Mechanistic Overview of the Immune Evasion, Angiogenesis and Diagnostic Applications in Cancer
Vijetha Karen Kitchley
International PhD program in cell therapy and regenerative medicine, Taipei Medical University
Exosomes are nano-sized extracellular vesicles (30–150 nm) encapsulated by a phospholipid bilayer and secreted by both prokaryotic and eukaryotic cells to facilitate intercellular communication and molecular signaling (Fig. 1) [1]. Initially thought to function solely as cellular waste disposal vehicles, exosomes are now recognized as vital mediators of intercellular transport, delivering proteins, metabolites, and nucleic acids to various tissues [2, 3].
The formation of exosomes involves a multi-step process along the endosomal pathway. It begins with endocytosis, during which the plasma membrane invaginates to form early endosomes. These later mature into late endosomes [1]. During this maturation, cytoplasmic components such as proteins, lipids, and nucleic acids are sequestered into intraluminal vesicles (ILVs). These vesicles may then either fuse with the plasma membrane to release exosomes into the extracellular environment or be targeted for degradation via lysosomes if their contents are deemed non-essential [2].
Role of Exosomes in Cancer
Tumor Microenvironment (TME)
Exosomes significantly contribute to cancer development by modulating the tumor microenvironment (TME), largely through intercellular signaling. The bioactive molecules within exosomes are crucial for reprogramming the TME to favor tumor growth and progression [4]. For example, delta-like 4 protein (DLL4), transported via exosomes, induces angiogenic sprouting and enhances tumor aggressiveness in colorectal cancer [5, 6]. Tissue-specific targeting of exosomes is partly mediated by integrins, which are also involved in establishing the pre-metastatic niche and organ-specific metastasis in breast cancer [7]. Moreover, exosomal TGF-β promotes the differentiation of fibroblasts and mesenchymal stem cells into myofibroblasts, enhancing proliferation and invasiveness in prostate cancer [8, 9].
The TME is composed of various cellular constituents—endothelial cells, fibroblasts, immune cells—that collectively influence tumor progression [10]. Tumor-derived exosomes (TDEs) play a central role in regulating immune cell recruitment and function within this environment [11]. TDEs modulate the differentiation and activity of key immune cells, including NK cells, macrophages, T-cells, and B-cells [12]. Furthermore, exosomes from cancer cells can activate stromal fibroblasts into cancer-associated fibroblasts (CAFs), promoting metastasis and tumor invasion [13].
Angiogenesis
Angiogenesis, the formation of new blood vessels, is crucial for tumor expansion and the development of metastatic sites [14]. Numerous studies have demonstrated that exosomes enhance angiogenesis by transferring pro-angiogenic factors. In head and neck squamous cell carcinoma (HNSCC), TGF-β-enriched TDEs are instrumental in driving angiogenic processes within the TME [15]. In nasopharyngeal carcinoma, exosomes are rich in ICAM-1, CD44v5, and MMP-13, while anti-angiogenic proteins like TSP-1 are downregulated [16, 17]. Bladder cancer-derived exosomes have shown overexpression of EDIL-3, a protein critical for vascular development and angiogenesis [18].
Immune Modulation, Inflammation, and Evasion
Cancer cells often evade immune surveillance, and exosomes contribute significantly to this process. TDEs are key mediators of immunomodulation, influencing inflammatory responses and immune evasion. They transport cytokines and other small proteins that regulate inflammation and immune cell behavior. Chronic inflammation, often sustained by TDEs, suppresses cytotoxic T-cell activity and facilitates tumor progression [19, 20]. These vesicles can inhibit the differentiation and maturation of monocytes, contributing to an immunosuppressive microenvironment. This effect is largely dependent on exosomal proteins such as TGF-β, IL-6, and prostaglandin E2 (PGE2) [21]. IL-6 secreted via the PI3K/AKT/mTOR pathway also suppresses the differentiation of myeloid precursors in the bone marrow. Additionally, exosomal TGF-β1 can impair dendritic cell maturation [22].
Therapy Resistance
Exosomes have been implicated in modulating cell death pathways in recipient cells, thereby contributing to therapy resistance. These vesicles may originate from either viable or apoptotic cells. For example, fibroblast-derived exosomes containing membrane-bound TNF-α have been shown to suppress activation-induced cell death (AICD) in CD4+ T-cells [23], while exosomes bearing Fas ligand can trigger AICD in T-cells. In neuroblastoma, exosomes from N-myc-amplified cells enhance the survival of non-amplified cells by promoting resistance to doxorubicin [24]. In colorectal cancer, apoptotic vesicles released by tumor cells can induce T-cell apoptosis [25]. TDEs can also contain anti-apoptotic proteins such as survivin, XIAP, cIAP1, and cIAP2, helping cancer cells evade programmed cell death [26]. In bladder cancer, resistance is further reinforced by exosomal upregulation of Bcl-2 and cyclin D1, alongside the suppression of Bax and caspase-3 [27].
Diagnostic and Theranostic Applications
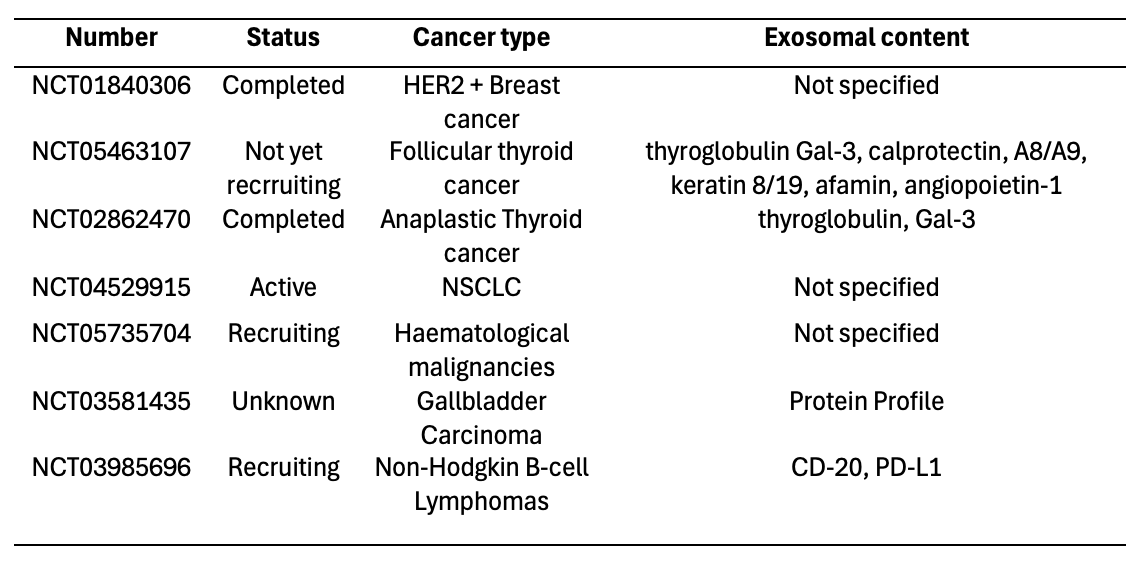
Accurate cancer diagnosis, risk assessment, and effective screening rely heavily on high-quality biomarkers. Tumor-derived exosomes have emerged as promising non-invasive diagnostic tools. Table 1 lists ongoing clinical trials evaluating exosomal proteins as biomarkers.
Table 1. Clinical trials focused on exosomal proteins as cancer biomarkers. [28]
Conclusion
Exosomes participate in a wide range of biological processes essential to cancer progression, including extracellular matrix remodeling, angiogenesis, immune suppression, metastasis, and therapy resistance. Their cargo acts as a key communication tool within the TME and serves as a valuable source of biomarkers for cancer diagnosis, prognosis, and treatment. The utility of exosomes in liquid biopsies presents an alternative to traditional tissue biopsies. Ongoing research continues to uncover specific roles and mechanisms of exosomes in various cancer types, paving the way for more personalized and effective cancer therapies.
- Minciacchi, V.R., M.R. Freeman, and D. Di Vizio, Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol, 2015. 40: p. 41-51.
- Grant, B.D. and J.G. Donaldson, Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol, 2009. 10(9): p. 597-608.
- Xie, S., Q. Zhang, and L. Jiang, Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes, 2022. 12(5): p. 498.
- Tai Y, Chen K, Hsieh J, Shen T. Exosomes in cancer development and clinical applications. Cancer Sci. 2018;109:2364–74.
- Sheldon H, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–94.
- Zhang Z, et al. Delta-like ligand 4 level in colorectal cancer is associated with tumor aggressiveness and clinical outcome. Cancer Biomark Sect Dis Markers. 2022;33:415–22.
- Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35.
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30.
- Chowdhury R, et al. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts.Oncotarget. 2015;6:715–31.
- Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18:32.
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contex- ture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306.
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93.
- LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018;11:dmm029447.
- Katayama Y, et al. Tumor neovascularization and developments in therapeutics. Cancers. 2019;11:316.
- Ludwig N, Yerneni SS, Razzo BM, Whiteside TL. Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Mol Cancer Res. 2018;16:1798–808.
- Chan Y-K, et al. Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int J Cancer. 2015;137:1830–41.
- You Y, et al. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106:1669–77.
- Beckham CJ, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014;192:583–92.
- Lim S-O, et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–39.
- Grinberg-Bleyer Y, Ghosh S. A novel link between inflammation and cancer. Cancer Cell. 2016;30:829–30.
- Yu S, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells1. J Immunol. 2007;178:6867–75.
- Wang M, Zhang B. The immunomodulation potential of exosomes in tumor microenvironment. J Immunol Res. 2021;2021:e3710372.
- Zhang H-G, et al. A membrane form of TNF-α presented by exosomes delays T cell activation-induced cell death1. J Immunol. 2006;176:7385–93.
- Sanwlani R, Gangoda L. Role of extracellular vesicles in cell death and inflammation. Cells. 2021;10:2663.
- Huber V, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–804.
- Valenzuela MMA, et al. Exosomes secreted from human cancer cell lines contain Inhibitors of Apoptosis (IAP). Cancer Microenviron Off J Int Cancer Microenviron Soc. 2015;8:65–73.
- Yang L, Wu X-H, Wang D, Luo C-L, Chen L-X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep. 2013;8:1272–8.
- Hánělová, K., Raudenská, M., Masařík, M. et al. Protein cargo in extracellular vesicles as the key mediator in the progression of cancer. Cell Commun Signal 22, 25 (2024).
Leave a comment