Junko Matsuo & Setsuko Hashimoto
CellSeed Inc., Tokyo, Japan
Abstract
Cell sheet engineering has emerged as a powerful approach for cell therapy including cartilage regeneration. We summarize the feature of cell sheet using temperature-responsive cell cultureware, and its clinical application and highlight the regulatory frameworks of regenerative medicine in Japan. We also introduce our allogeneic chondrocyte sheet (CLS2901C), which is currently undergoing a Phase III clinical trial. We describe our experience overcoming practical challenges in developing the allogeneic chondrocyte sheet for approval under the Act on Pharmaceuticals and Medical Devices (PMD Act) in Japan.
Keywords:
Cell Sheet Engineering, Cartilage Regeneration, Allogeneic Chondrocyte Sheet, Japan Regulatory Framework
Regulatory Framework for Regenerative Medicines in Japan
Japan has two laws that govern the implementation of regenerative medicine (Figure 1). For development of marketing authorization purpose, a clinical trial is conducted under the Act on Pharmaceuticals and Medical Devices (PMD Act). As of August 2025, 23 regenerative medical products have been approved. Considering the specific features of cell therapy products, an early approval system (Conditional and Time-Limited Approval) has been established under the PMD Act. Six products have been approved using the system, yet no products have received full approval. For the technologies whose efficacy and safety have not yet been established, Act on the Safety of Regenerative Medicine (Safety Act) can be applied. For example, clinical research mainly conducted by academia, is regulated by the Safety Act. Advanced Medical Care B (corresponding to the law to the Regulation Governing the Application of Specific Medical Examination Technique and Medical Device in Taiwan) is also regulated by the Safety Act. Private insurance offers an option to cover the cost of Advanced Medical Care B. The Safety Act allowed outsourcing of cell manufacturing, thus enabling CDMO business in Japan.
Cell Sheet Engineering
Achieving regenerative medicine requires interdisciplinary research across many fields, especially engineering, to prepare cells as cell therapy products. In general, harvesting the adherent cells intact is difficult. Traditionally, cells are harvested using proteolytic enzymes or physical methods such as scraping, which could damage the cells. To solve this problem, Professor Teruo Okano of Tokyo Women’s Medical University pioneered the world-first “cell sheet engineering” by introducing temperature-responsive polymer on the surface of the cell cultureware with Nano-Bio Interface technology [1].
This breakthrough innovation was strongly supported by Japanese funding agencies. After cells reach confluence at 37°C, the cells can be detached as intact sheet without damage simply by reducing the temperature to 20°C. These sheet retain cell-cell junctions, adhesive proteins, the extracellular matrix, imparting adhesive characteristics to biological tissues [2].
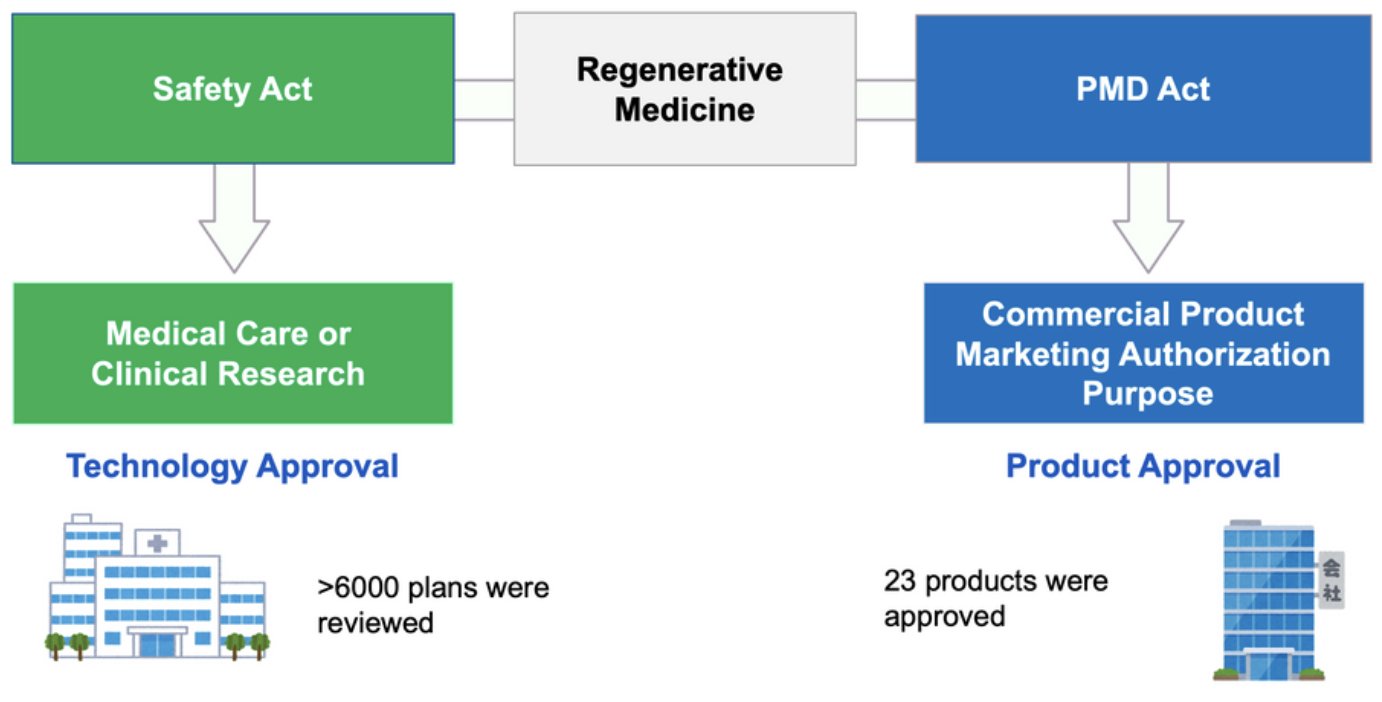
Figure 1. Regulatory framework for Regenerative Medicine in Japan
These are critical to maintain cellular functions and promoting tissue regeneration, and suitable for applications in cell therapy. CellSeed Inc. was established to commercialize “cell sheet engineering”. CellSeed is currently engaged in development of regenerative medicine, marketing of temperature-responsive cultureware and the CDMO business. “Cell sheet engineering” is progressing in various clinical applications to cover unmet medical needs (Figure 2), and among 23 approved regenerative medicine products in Japan, three products applied cell sheet engineering.
Cell Sheet Therapy for Cartilage Regeneration
Osteoarthritis, caused by obesity, aging, genetics, occupation, sports, or trauma, is increasing in an aging society. There are an estimated 25 million potential patients in Japan, with 8 million symptomatic cases. Although it is an important disease that should be addressed when considering long-term care costs and medical expenditure, fundamental therapy has not yet been established. Dr. Masato Sato of Tokai University applied cell sheet engineering for cartilage regeneration using chondrocyte sheets. Clinical research revealed the efficacy and safety of autologous chondrocyte sheet. Transplantation of autologous chondrocyte cell sheets along with open-wedge high tibial osteotomy (OWHTO) promoted hyaline cartilage repair [3].
Another clinical research showed that the transplantation of polydactyly-derived allogeneic chondrocyte sheets along with OWHTO improved clinical symptoms with no serious adverse events. The histological examination confirmed regeneration of hyaline cartilage [4]. Allogeneic chondrocyte sheet can eliminate the need for invasive tissue collection, remove restrictions on the knee’s damaged area, and ensure a stable cell sheet supply, making treatment available to more patients.
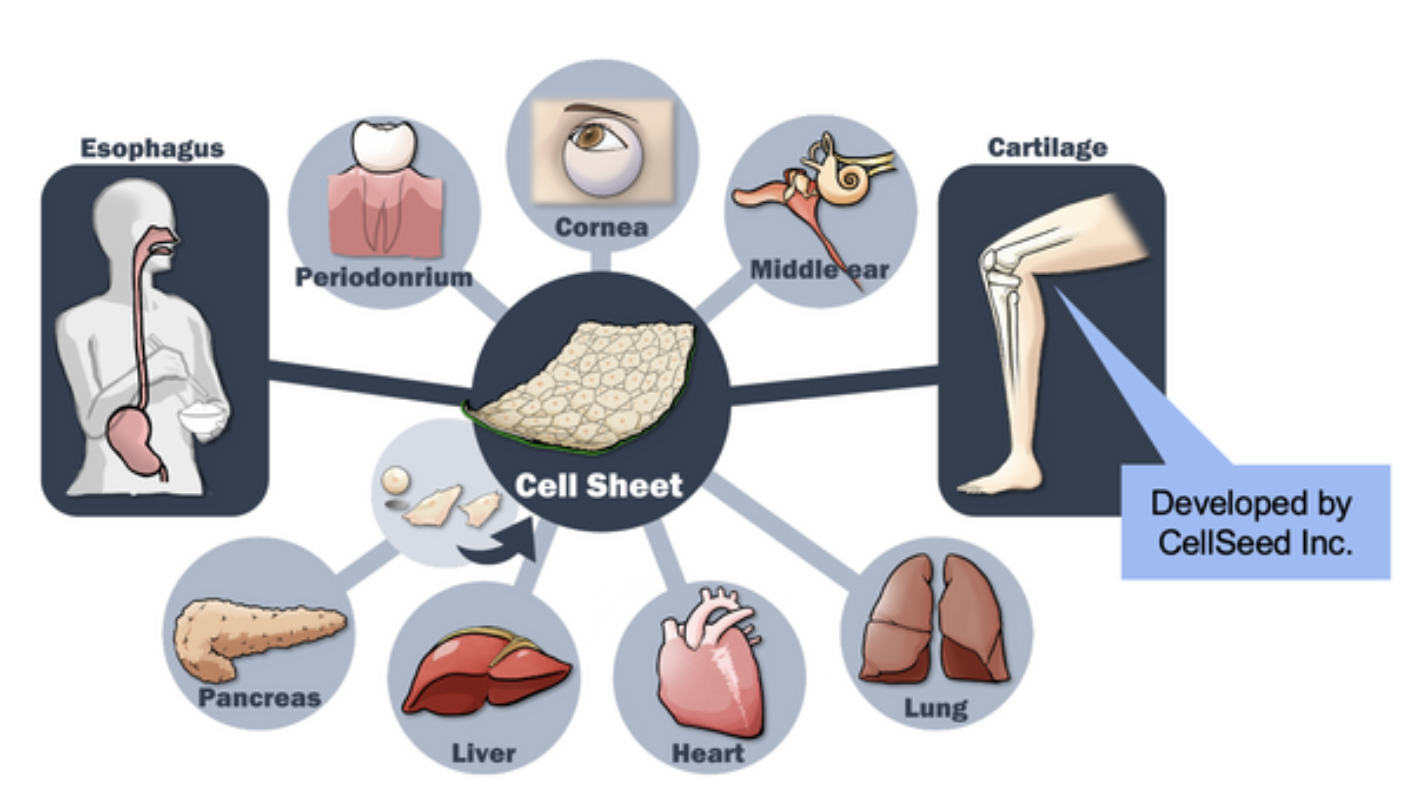
Figure 2. “Cell Sheet Engineering” for Regenerative Medicine
Product Development by CellSeed Inc.
After technology transfer from Tokai University, CellSeed is developing an allogeneic chondrocyte sheet (CLS2901C) to obtain approval under PMD Act in Japan. Since allogeneic cells use excised tissue from patients with polydactyly (extra fingers or toes) as raw materials (Figure 3), it is necessary to address ethical issues. In December 2020, the Ethics Committee of the National Center for Child Health and Development (NCCHD) approved the collection of cartilage tissue from patients with polydactyly for commercial use. This process took approximately two years.
Furthermore, in August 2022, NCCHD and CellSeed reached an agreement for the constant supply and commercial use of excised tissue from polydactyly surgeries. With this agreement, it became possible to obtain the human tissue required for clinical trials and marketing of allogeneic chondrocyte sheet. We established a master cell bank (MCB) of chondrocytes by confirming safety and efficacy. After several consultation with PMDA, we submitted a notification of Phase III clinical trial in Japan in September 2023. For donor selection for MCB, we evaluate the efficacy on cartilage regeneration using the nude rat osteochondral defect model. Although this model is useful in that it enables direct efficacy assessment, it is time-consuming, expensive and has animal welfare issues.

Figure 3. Manufacturing process of Allogenic Chondrocyte Sheet (CLS2901C)
Therefore, we analyzed potential in vitro markers to predict efficacy on cartilage regeneration. We evaluated the correlation between in vivo tissue repair potency scored by histological grading system and the omics data derived from the cell sheet. We extracted several molecules that were highly correlated with in vivo efficacy. In the future, we may replace animal testing and improve quality testing with these markers.
Challenges to Practical Implementation
When companies procure raw materials from medical institutions, various extra, uncompensated tasks arise for the medical institutions. On the company side, the search for and negotiation with cooperating medical institutions is also necessary, which further delays product development. Regenerative medicine products are a new modality with the potential to transform existing treatment concepts. Unlike conventional pharmaceuticals, there are many differences with these products, such as product diversity, unique manufacturing processes and quality control, and an extremely short shelf life. As current regulations may not fully accommodate this new modality, it is crucial to review and update rules to adapt regenerative medicine products.
The Forum for Innovative Regenerative Medicine (FIRM) was incorporated in 2011 to establish social systems that ensure safe and stable access to the benefits of regenerative medicine (https://firm.or.jp/en/). FIRM consists of approximately 200 companies involved in regenerative medicine. FIRM lobbies for improvement of treatment access in consideration of the unique characteristics of regenerative medicine products. For example, approved autologous cell products derived from the patient exhibit variability in raw material quality, which can lead to the production of out-of-specification (OOS) products. OOS products are sometimes administered under compassionate grounds, within the framework of clinical trials in Japan. Although this approach is ethical, it imposes significant operational and administrative burdens on medical institutions and marketing authorization holders, raising concerns about sustainability [5]. The revision to the PMD Act in 2025 introduced an exceptional provision.
Under this measure, the sales and supply of OOS products using autologous cells are permitted solely when certain criteria are fulfilled: product safety is assured, patient needs are considered, and physicians recognize the product’s value.
Conclusion
This article introduces the regulatory framework for regenerative medicines in Japan and cell sheet engineering, as well as its applications in cell therapy,
including cartilage regeneration. Regenerative medicine products are a new modality with the potential to transform existing treatment concepts. Our allogeneic chondrocyte sheet is also a promising cell therapy product with the potential to treat osteoarthritis at its root.
Many stakeholders are making dedicated efforts towards practical implementation for regenerative medicine. There are many challenges to be addressed, but we hope to resolve these issues through collaboration among industry, regulatory authorities, and academia. Our goal is to deliver better healthcare to the patients.
Acknowledgements
We sincerely thank Dr. Masato Sato (Tokai University), Dr. Akihiro Umezawa, and Dr. Kazuaki Nakamura (National Center for Child Health and Development) for their valuable advice and support.
The development of CLS2901C was supported by the Japan Agency for Medical Research and Development (AMED).

Setsuko Hashimoto, Ph.D.
Representative Board Director,
President/CEO, CellSeed Inc.
Setsuko is President and CEO of CellSeed Inc. She has over 30-year commercial experience in the bio-industry before joining CellSeed. Dr. Hashimoto is well networked globally to academia, government and industry.

Corresponding author: junko.matsuo@cellseed.com
Junko is the Head of Cell Sheet CMC Development at CellSeed Inc.
She has about 15 years of experience in cardiac electrophysiological safety testing, utilizing gene-expressing cells, primary myocytes, and iPS cell-derived myocytes. Before joining CellSeed. Inc., she contributed to business development of pre-clinical services in Taiwan.
References
- Okano T, et al. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16(4):297–303.
- Shimizu T. Cell sheet-based tissue engineering for fabricating 3-dimensional heart tissues. Circ J. 2014;78:2594–2603.
- Sato M, et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen Med. 2019;4:4.
- Hamahashi K, et al. Polydactyly derived allogeneic chondrocyte cell-sheet transplantation with high tibial osteotomy as regenerative therapy for knee osteoarthritis. NPJ Regen Med. 2022;7(1):71.
- Sasai M, et al. Challenges and opportunities in the compassionate use of out-of-specification products in autologous regenerative medicine. Stem Cell Res Ther. 2025;16:238.
- Forum of Innovative Regenerative Medicine: https://firm.or.jp/en/
Leave a comment