Masato Sato
Department of Orthopaedic Surgery, School of Medicine, Tokai University
Center for Musculoskeletal Innovative Research and Advancement (C-MIRA), Tokai University Graduate School
Tokai University Institute of Medical Sciences
Abstract
Cartilage cell‑sheet engineering on temperature‑responsive culture surfaces preserves the extracellular matrix (ECM) and enables the sheet to adhere directly and uniformly to chondral defects without a scaffold. As a joint‑preserving, disease‑modifying approach for osteoarthritis of the knee (OAK), we have advanced both autologous and allogeneic cartilage cell‑sheet transplantation from preclinical studies to clinical investigations. Here, we outline methods, outcomes, and the mechanistic basis that support clinical translation.
Background
OAK is a slowly progressive degenerative disease for which disease‑modifying, joint‑preserving therapies are needed. Cartilage cell sheets fabricated on temperature‑responsive culture dishes retain ECM and attach to defects as a ‘sheet,’ allowing firm coverage of the lesion. In preclinical models, chondrocyte sheets outperformed synovial cell sheets in a rabbit osteochondral defect model; in immunodeficient rats, human chondrocyte sheets induced hyaline‑like cartilage regeneration, improved International Cartilage Repair Society (ICRS) histological scores versus controls, and ameliorated pain‑related behavior. Efficacy was also confirmed in a large‑animal (mini‑pig) model.
Methods
Clinically, we first initiated autologous cartilage cell‑sheet transplantation in 2013. Building on that experience, we began in 2017 a single‑arm, open‑label prospective study using allogeneic sheets prepared from polydactyly surgical discards—administered without immunosuppression. The latter combined open‑wedge high tibial osteotomy (OWHTO) with bone marrow stimulation (microfracture or abrasion technique) and allogeneic sheet transplantation (the RMSC approach) in 10 patients with Outerbridge grade III–IV OAK. Endpoints included patient‑reported outcomes (KOOS and Lysholm Knee Score), MRI‑based repair assessment (MOCART 2.0), viscoelastic properties measured by laser‑induced photoacoustics (LIPA),
and arthroscopic biopsy. The autologous program was accepted in Japan as Advanced Medical Care B in 2019 for further clinical use.
Results
Autologous sheets were transplanted in 20 cases under the Advanced Medical Care B framework, with analyses underway. In the allogeneic cohort, correction of lower‑limb alignment was accompanied by improvement in MOCART 2.0 scores. LIPA measurements showed viscoelasticity of the repair tissue approaching that of native cartilage within the same joint. The mean thickness of regenerated cartilage was 3.54 mm. Arthroscopic biopsies at hardware removal showed strong Safranin O staining and COL2 positivity. No serious adverse events were observed. Cells derived from polydactyly tissue exhibited robust proliferation and laminar sheet formation, with surface markers and anabolic factor production comparable to autologous cells, supporting a stable supply chain for an allogeneic regenerative medicine product. An industry‑sponsored clinical trial of the allogeneic sheet has been initiated.
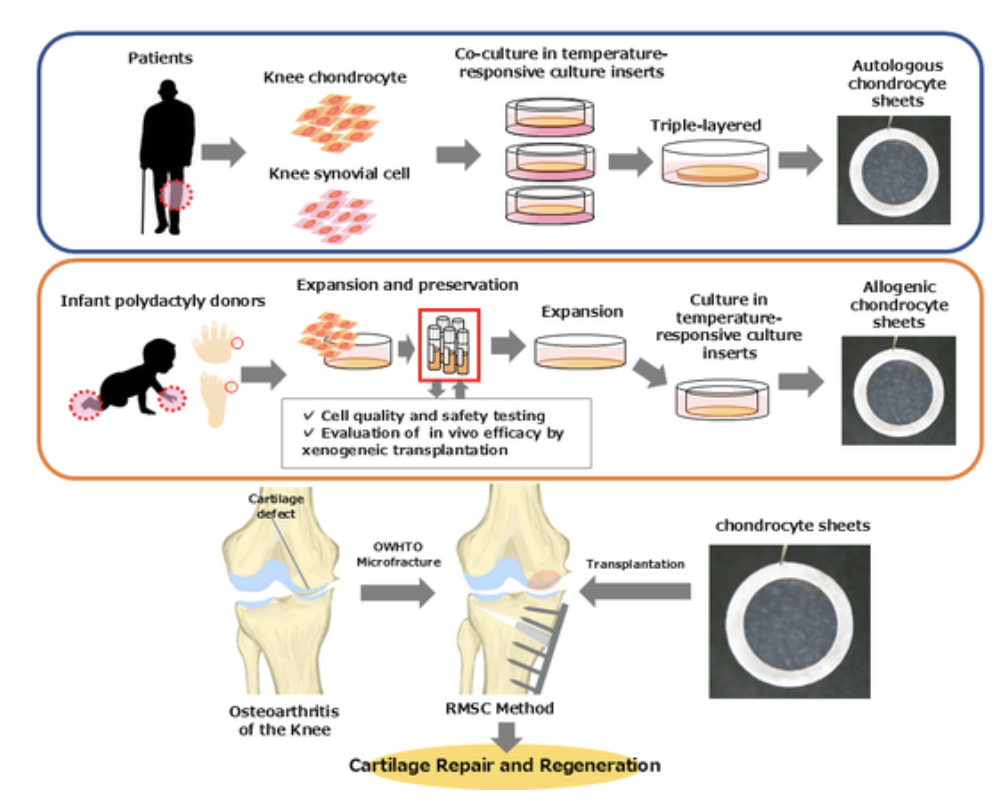
Figure 1. Concept and clinical workflow for autologous and allogeneic cartilage cell sheets. The schematic summarizes sheet fabrication, transplantation alongside OWHTO and marrow stimulation, and multimodal assessments (KOOS/Lysholm, MOCART 2.0 MRI, LIPA viscoelasticity, and arthroscopic biopsy).
Mechanism of Action
Omics‑level analyses identified gene sets correlated with clinical outcomes. TGFB1 correlated with KOOS/Lysholm, whereas ESM1 and ACKR4 correlated with histologic scores (OARSI/ICRS II). These factors relate to ECM organization and angiogenic regulation and may serve as biomarkers for donor selection and quality standardization. Preclinically, secreted factors from the sheets—including TGFβ1 and MIA—likely contribute to the pro‑regenerative niche.
Conclusions
Cartilage cell‑sheet transplantation combines planar adhesion and ECM preservation with paracrine activity. When coupled with alignment correction procedures such as OWHTO, it can improve both symptoms (KOOS/Lysholm) and structure (MOCART 2.0, OARSI/ICRS II), making it a promising disease‑modifying, joint‑preserving therapy for OAK. While current clinical datasets derive from small, single‑arm studies, implementation of biomarker‑guided donor selection and quality control is paving the way for practical deployment as an allogeneic regenerative medicine product.
Keywords:
Osteoarthritis of the knee, cartilage cell sheet, allogeneic transplantation, autologous transplantation, open‑wedge high tibial osteotomy (OWHTO), MOCART 2.0, laser‑induced photoacoustics (LIPA).

Masato Sato, MD, PhD, is Professor and Director with the Department of Orthopaedic Surgery, School of Medicine, Tokai University; the Center for Musculoskeletal Innovative Research and Advancement (C-MIRA), Tokai University Graduate School; and the Tokai University Institute of Medical Sciences. His research focuses on musculoskeletal regenerative medicine and cartilage repair using cell‑sheet engineering.
References:
- Kaneshiro N, Sato M, Ishihara M, Mitani G, Sakai H, Mochida J. Biochemical and Biophysical Research Communications 349(2): 723–731.
- Ito S, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, Ukai T, et al. Biomaterials 33(21): 5278–5286.
- Takizawa D, Sato M, Okada E, Takahashi T, Maehara M, Tominaga A, et al. Journal of Tissue Engineering and Regenerative Medicine 14(9): 1296–1306.
- Ebihara G, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, Ito S, Ukai T, et al. Biomaterials 33(15): 3846–3851.
- Sato M, Yamato M, Mitani G, Takagaki T, Hamahashi K, Nakamura Y, et al. NPJ Regenerative Medicine 4(1): 4.
- Hamahashi K, Toyoda E, Ishihara M, Mitani G, Takagaki T, Kaneshiro N, et al. NPJ Regenerative Medicine 7(1): 71.
- Maehara M, Sato M, Toyoda E, Takahashi T, Okada E, Kotoku T, et al. Inflammation and Regeneration 37(1): 22.
- Kokubo M, Sato M, Yamato M, Mitani G, Uchiyama Y, Mochida J, Okano T. Journal of Tissue Engineering and Regenerative Medicine 11(10): 2885–2894.
- Hamahashi K, Sato M, Yamato M, Kokubo M, Mitani G, Ito S, Nagai T, et al. Journal of Tissue Engineering and Regenerative Medicine 9(1): 24–30.
Leave a comment